Instruments
CAB instruments are to be found at two locations of the University of Copenhagen:
North Campus
 |
High-content screening spinning discRobot microscope for high throughput imaging and screening. Liquid for assays and environmental control are available. |
 |
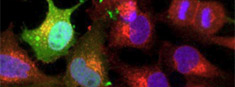
Upright point-scanning confocalLive imaging of cells and simple tissues for (co-)localization studies of fluorochromes and fluorescent protein, FRET, FRAP |
 |
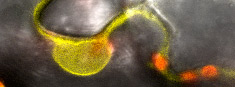
High-end confocal for FLIMLive imaging of cells, tissue and organs, protein localization, intracellular ion concentrations, FRAP, FRET, FLIM |
 |
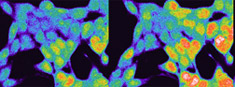
BioStationVisualization of processes in living cell over extended periods of time; cell cycle, cell differentiation, migration, cell-cell interaction |
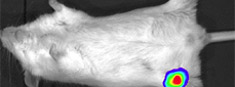 |
Whole animal imagerIn vivo imaging of mice; in vivo cancer experiments with bioluminescent or fluorescent cells; disease models |
Frederiksberg Campus |
|
High-end inverted CLSMExtremely versatile for many applications including live imaging of cells and tissues, localisation studies, multi-dimensional imaging (3D, time series, spectral scans...) and many others. |
|
Inverted spinning disk confocalState-of-the-art Spinning Disk Confocal Microscope for high speed, high sensitivity confocal imaging. With optional vertical stage. |
|
 |
Super resolution microscopeSuperresolution (3D-SIM, TIRF and PALM/STORM) for protein immunolocalisation and GFP-fusion proteins |
 |
High-end point CLSMLive imaging of cells and tissues, colocalisation studies, 4D confocal imaging (xyzt), (xyΛ2), photoactivaiton, FRET, FRAP |
 |
Fluorescence Lifetime MicroscopeInteraction studies (FLIM-FRET), analysis of diffusion dynamics (FCS), separation of fluorophores with similar spectral properties |
 |
Inverted point CLSMLive imaging of animal cells and tissues, (co-) localisation of fluorochromes and fluorescent proteins, FRET, FRAP |
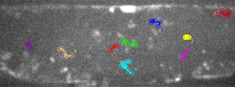 |
Spinning disk confocalLive imaging of ion and pH homeostasis, fluorescence cinematography, 4D confocal imaging (xyzt), FRET, FRAP |
 |
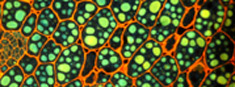
Histology laboratoryPreparation of fresh and fixed material, histology and immunofluorescence for light, fluorescence and electron microscopy |
 |
Research microscopesTwo dissection and six wide fields microscopes allowing detection of fluorescent reporters and dyes |
 |
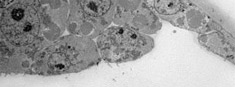
Scanning electron microscopeHigh resolution imaging of the surface of cells, tissues and organs for fixed/goldsputtered specimens but also for low vacuum ESEM |
 |
High resolution TEMNegative contrast (proteins, nucleic acid or virus) and ultrathin sections of fixed bacteria or eukaryotes and immunolocalisation |
 |
Raman microscopeIdentification and visualization of the distribution of chemical compounds within samples. |
 |
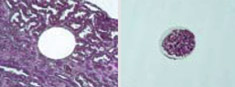
Laser ablation ICP-MSAblation from soft tissues; multi-elemental bioimaging; multi-plexed quantitative proteomics using lanthanide tagged antibodies |
 |
Laser microdissectionCollection of genetic and metabolite contents from single cells or small cell groups for RT-PCR and mass spectrometry |
Other imaging centers in Denmark
Core Facility for Integrated Microscopy - CFIM
Department of Biomedical Sciences
University of Copenhagen
Bioimaging Core Facility, AU
Department of Biomedicine, Aarhus University
Danish Molecular Biomedical Imaging Center - DaMBIC
University of Southern Denmark
DTU Nanolab - National Centre for Nano Fabrication & Characterization
Technical University of Denmark
3D Imaging Centre (3DIM)
Danish Science hub for neutron and X-ray imaging, Technical University of Denmark
